Boiling Point Diagram (TXY Diagram)
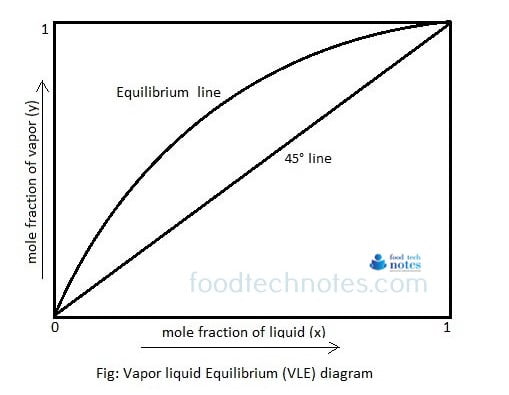
Equilibrium diagram (X-Y) diagram is simple diagram than boiling point diagram in which plot of temperature is eliminated. The boiling point diagram represents dynamic equilibrium of mole fraction of vapor and liquid phase at certain temperature at constant pressure. Boiling point diagram at constant pressure for binary mixture having component A and B is as below.